2023-09-25 マサチューセッツ大学アマースト校
◆この細胞の記憶はワクチンの仕組みの基礎です。細胞の記憶を強化して腫瘍を認識させることは、がん治療を改善するのに役立つ可能性があり、次世代のがん免疫療法の新戦略を示唆しています。この研究は、がん細胞を認識する細胞の記憶を向上させる新しい戦略を提供し、がん治療の革命をもたらす可能性があります。
<関連情報>
- https://www.umass.edu/news/article/researchers-umass-amherst-show-how-small-strand-rna-key-fighting-cancer
- https://www.nature.com/articles/s41467-023-40959-7
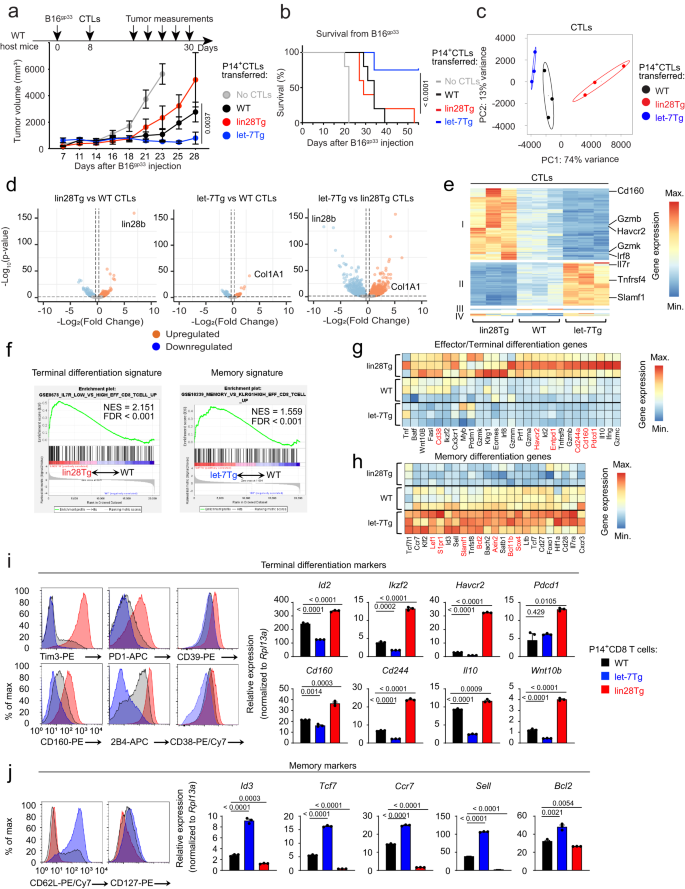
Let-7は記憶を促進し終末分化に拮抗することによりマウス抗腫瘍CD8 T細胞応答を増強する Let-7 enhances murine anti-tumor CD8 T cell responses by promoting memory and antagonizing terminal differentiation
Alexandria C. Wells,Kaito A. Hioki,Constance C. Angelou,Adam C. Lynch,Xueting Liang,Daniel J. Ryan,Iris Thesmar,Saule Zhanybekova,Saulius Zuklys,Jacob Ullom,Agnes Cheong,Jesse Mager,Georg A. Hollander,Elena L. Pobezinskaya & Leonid A. Pobezinsky
Nature Communications Published:11 September 2023
DOI:https://doi.org/10.1038/s41467-023-40959-7
Abstract
The success of the CD8 T cell-mediated immune response against infections and tumors depends on the formation of a long-lived memory pool, and the protection of effector cells from exhaustion. The advent of checkpoint blockade therapy has significantly improved anti-tumor therapeutic outcomes by reversing CD8 T cell exhaustion, but fails to generate effector cells with memory potential. Here, using in vivo mouse models, we show that let-7 miRNAs determine CD8 T cell fate, where maintenance of let-7 expression during early cell activation results in memory CD8 T cell formation and tumor clearance. Conversely, let-7-deficiency promotes the generation of a terminal effector population that becomes vulnerable to exhaustion and cell death in immunosuppressive environments and fails to reject tumors. Mechanistically, let-7 restrains metabolic changes that occur during T cell activation through the inhibition of the PI3K/AKT/mTOR signaling pathway and production of reactive oxygen species, potent drivers of terminal differentiation and exhaustion. Thus, our results reveal a role for let-7 in the time-sensitive support of memory formation and the protection of effector cells from exhaustion. Overall, our data suggest a strategy in developing next-generation immunotherapies by preserving the multipotency of effector cells rather than enhancing the efficacy of differentiation.